Dr. Linus Pauling
Biological Significance
Vitamin C is purely the L-enantiomer of ascorbate; the opposite D-enantiomer has no physiological significance. Both forms are mirror images of the same molecular structure. When L-ascorbate, which is a strong reducing agent, carries out its reducing function, it is converted to its oxidized form, L-dehydroascorbate. L-dehydroascorbate can then be reduced back to the active L-ascorbate form in the body by enzymes and glutathione. During this process semidehydroascorbic acid radical is formed. Ascorbate free radical reacts poorly with oxygen, and thus, will not create a superoxide. Instead two semidehydroascorbate radicals will react and form one ascorbate and one dehydroascorbate. With the help of glutathione, dehydroxyascorbate is converted back to ascorbate. The presence of Glutathione is crucial since it spares ascorbate and improves antioxidant capacity of blood. Without it dehydroxyascorbate could not convert back to ascorbate.
L-ascorbate is a weak sugar acid structurally related to glucose which naturally occurs either attached to a hydrogen ion, forming ascorbic acid, or to a metal ion, forming a mineral ascorbate.
Biosynthesis
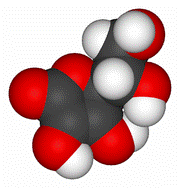
Model of a vitamin C molecule. Black is carbon, red is oxygen, and white is hydrogen
The vast majority of animals and plants are able to synthesize their own vitamin C, through a sequence of four enzyme-driven steps, which convert glucose to vitamin C. The glucose needed to produce ascorbate in the liver (in mammals and perching birds) is extracted from glycogen; ascorbate synthesis is a glycogenolysis-dependent process. In reptiles and birds the biosynthesis is carried out in the kidneys.
Among the animals that have lost the ability to synthesise vitamin C are simians (specifically the suborder haplorrhini, which includes humans), guinea pigs, a number of species of passerine birds (but not all of them), and many (perhaps all) major families of bats. These animals all lack the L-gulonolactone oxidase (GULO) enzyme, which is required in the last step of vitamin C synthesis, because they have a defective form of the gene for the enzyme (Pseudogene ΨGULO). Some of these species (including humans) are able to make do with the lower levels available from their diets by recycling oxidised vitamin C.
Most simians consume the vitamin in amounts 10 to 20 times higher than that recommended by governments for humans. This discrepancy constitutes the basis of the controversy on current recommended dietary allowances.
It has been noted that the loss of the ability to synthesize ascorbate strikingly parallels the evolutionary loss of the ability to break down uric acid. Uric acid and ascorbate are both strong reducing agents. This has led to the suggestion that in higher primates, uric acid has taken over some of the functions of ascorbate. Ascorbic acid can be oxidized (broken down) in the human body by the enzyme L-ascorbate oxidase.
An adult goat, a typical example of a vitamin C-producing animal, will manufacture more than 13 g of vitamin C per day in normal health and the biosynthesis will increase “many fold under stress”. Trauma or injury has also been demonstrated to use up large quantities of vitamin C in humans. Some microorganisms such as the yeast Saccharomyces cerevisiae have been shown to be able to synthesize vitamin C from simple sugars.
Absorption And Transport
Ascorbic acid is absorbed in the body by both active transport and simple diffusion. Sodium Dependent Active Transport – Sodium-Ascorbate Co-Transporters (SVCTs) and Hexose transporters (GLUTs) are the two transporters required for absorption. SVCT1 and SVCT2 imported the reduced form of ascorbate across plasma membrane. GLUT1 and GLUT3 are the two glucose transporters and only transfer dehydroascorbic acid form of Vitamin C. Although dehydroascorbic acid is absorbed in higher rate than ascorbate, the amount of dehydroascorbic acid found in plasma and tissues under normal conditions is low, as cells rapidly reduce dehydroascorbic acid to ascorbate. Thus, SVCTs appear to be the predominant system for vitamin C transport in the body.
SVCT2 is involved in vitamin C transport in almost every tissue, the notable exception being red blood cells which lose SVCT proteins during maturation. Knockout animals for SVCT2 die shortly after birth, suggesting that SVCT2-mediated vitamin C transport is necessary for life.
With regular intake the absorption rate varies between 70 to 95%. However, the degree of absorption decreases as intake increases. At high intake (12g), human body can absorb ascorbic acid as low as 16%; while, at low intake (<20 mg) the absorption rate could reach up to 98%.
Biological tissues that accumulate over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina. Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.

Leave a comment